Introduction+
Historical Background
Vacuum-assisted biopsy (VAB) of the breast is a minimally invasive diagnostic tool for breast interventions that has gained widespread acceptance as an alternative to surgical biopsy. VAB involves the use of a vacuum-powered probe and a specialized needle to remove tissue samples from the breast lesion under imaging guidance, allowing for accurate diagnosis with minimal tissue damage and scarring compared to surgical biopsy 1,2.
The history of breast biopsy procedures has seen numerous technological advancements aimed at achieving more accurate diagnoses while minimizing invasiveness. Prior to the advent of Vacuum-Assisted Biopsy (VAB), Fine Needle Aspiration (FNA) and Core Needle Biopsy (CNB) were the primary methods for obtaining breast tissue samples 3,4. However, both techniques had limitations, including issues related to under-sampling and false negatives, which could lead to inaccurate or uncertain diagnoses 2,5,6.
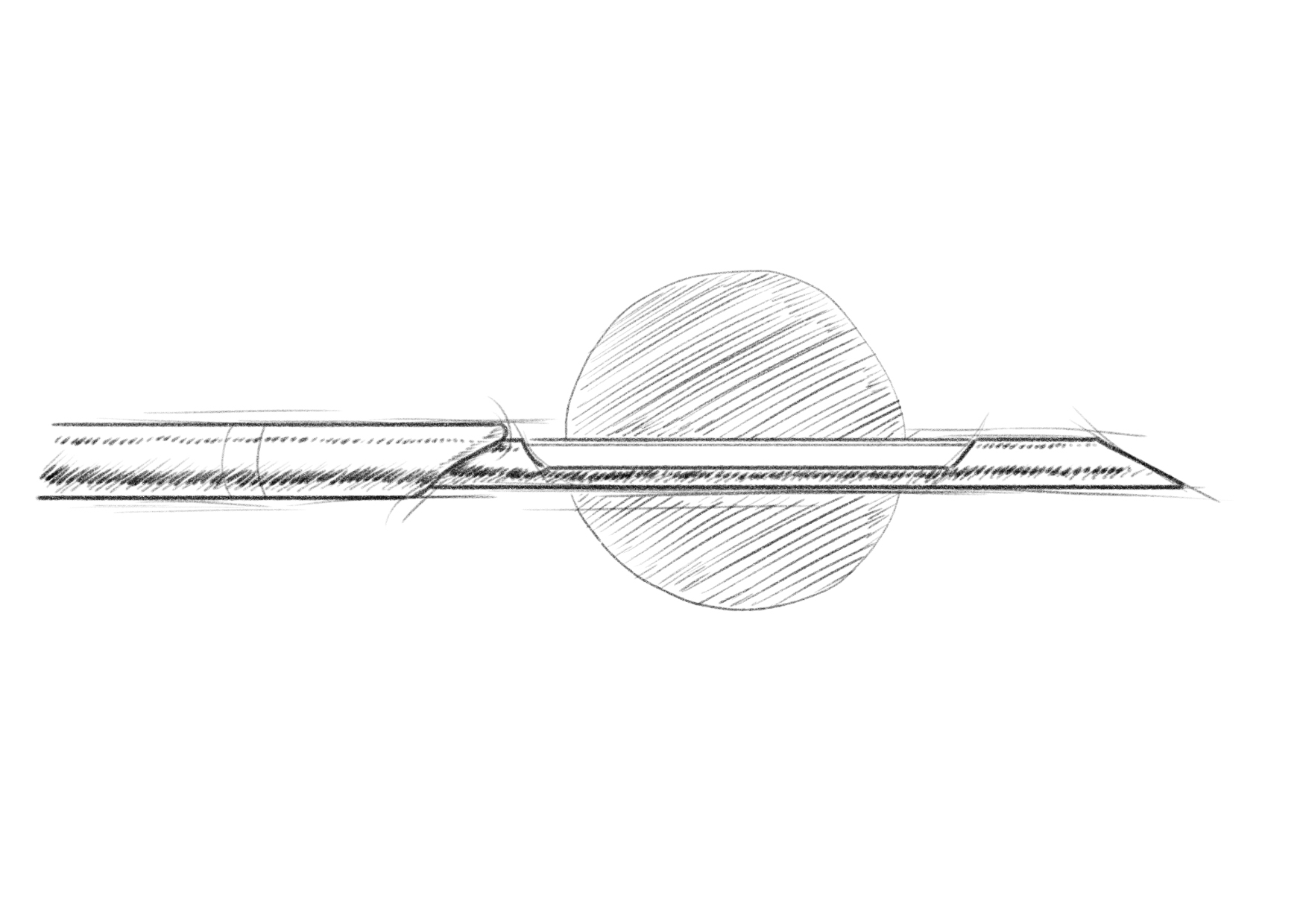
CNB
VAB
Recognizing these limitations and the need for a more accurate and less invasive alternative to surgical biopsy, VAB was developed during the mid-1990s. Two significant contributors to this field were Dr. Burbank and Dr. Parker, who in collaboration with a multidisciplinary team, developed a purpose-built stereotactic breast biopsy instrument in 1993 7, a 14-gauge needle with a coaxial system, specifically designed for tissue sampling. This marked a significant development in the evolution of breast biopsy techniques and the advent of vacuum-assisted biopsies. On August 5, 1994, the first stereotactic breast biopsy using this innovative device was performed at their center, a procedure subsequently referred to as “stereotactic mammotomy”7.
Early studies, such as that conducted by Parker et al. in 1997 5, demonstrated that VAB could accurately sample breast lesions while minimizing complications. Over the subsequent years, VAB has undergone further evolution in terms of needle design, imaging guidance, and overall performance. These advancements have resulted in the widespread acceptance of VAB as a standard diagnostic tool in breast interventions.
Equipment and Technique
The VAB equipment is specifically designed to facilitate accurate targeting and sampling of breast lesions. These devices can incorporate either wire or wireless guidance systems, which assist in guiding the biopsy needle to the intended target lesion.
Wired device
Wireless device
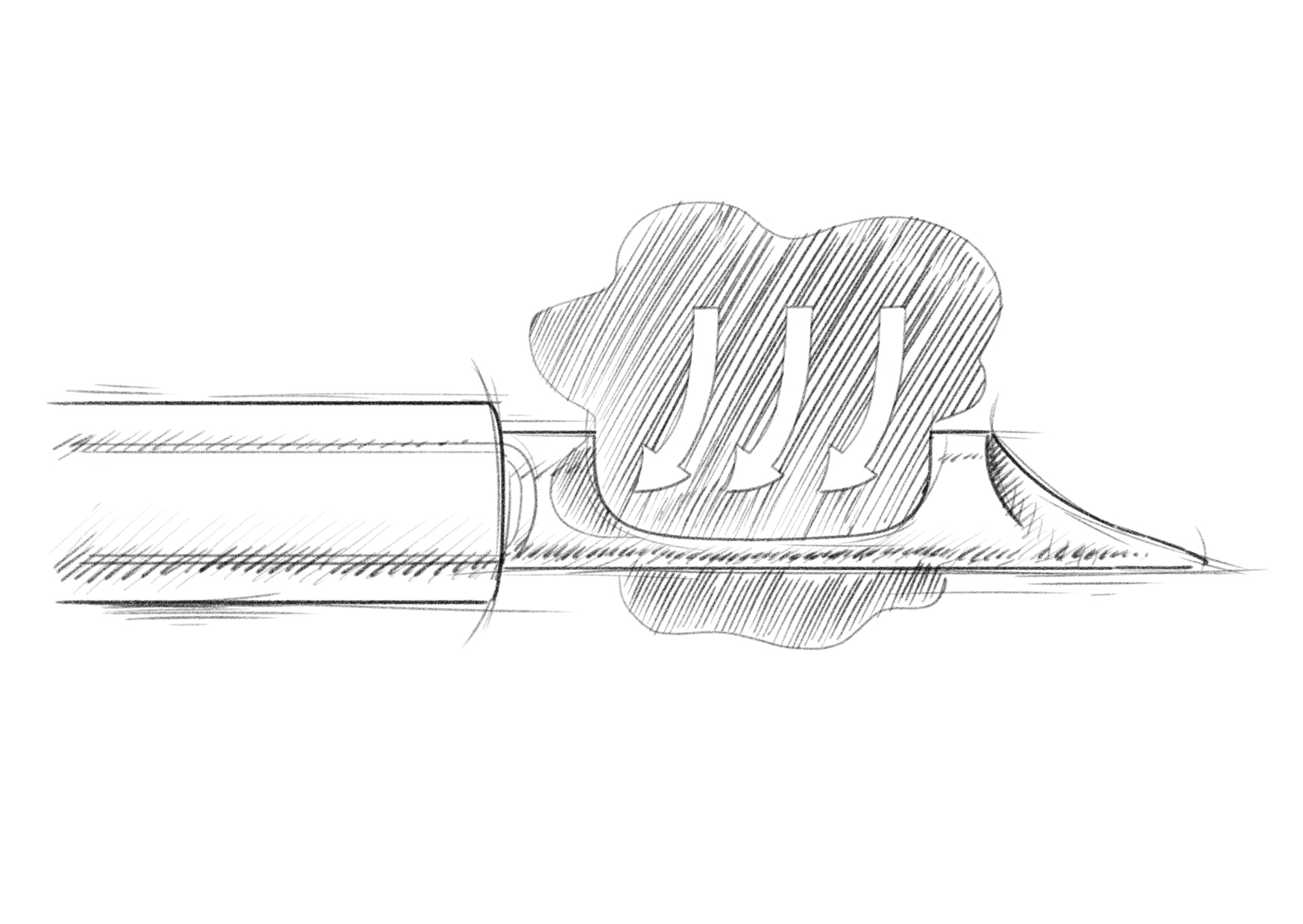
During a VAB, the procedure combines tissue aspiration and cutting mechanisms to collect tissue samples. The vacuum assistance within the VAB system plays a crucial role by creating suction within the needle. This vacuum suction draws the tissue into the needle aperture, providing stability during the sampling process. Once the tissue is drawn into the needle, a cutting mechanism is utilized to remove the tissue sample. The specific cutting mechanism may vary depending on the VAB device being used but commonly involves a rotating cutter or a similar mechanism within the needle. The cutter is activated to rotate or oscillate, effectively cutting through the tissue inside the needle and separating a sample from the surrounding tissue.
The integration of vacuum assistance and the cutting mechanism enables controlled and precise sampling of the tissue. The vacuum holds the tissue securely, minimizing the risk of sample contamination or displacement during the cutting process. The cutter ensures a clean and accurate separation of the tissue sample from the rest of the lesion, contributing to the overall success of the biopsy procedure.
Advantages
VAB provides superior control and precision during biopsy compared to automatic CNB devices. For instance, VAB eliminates needle tip excursion during the procedure, ensuring continuous operator control of the needle cannula within or beneath the targeted lesion. Moreover, the large sampling volume of the VAB cannula enables reliable sampling of small masses, which can be challenging with multiple CNB passes, especially for less experienced operators. VAB offers several additional advantages over CNB:
- Accuracy: VAB has been shown to provide more accurate diagnoses than CNB, with lower underestimation rates in cases with DCIS 8. The larger sample size obtained by VAB improves diagnostic accuracy and reduces the need for surgical excision in case of benign findings 9.
- Sample Size: Unlike CNB, which obtains a small tissue sample, VAB uses vacuum assistance to draw and cut a larger tissue sample, providing more material for pathological analysis 6.
- Reduction in Sampling Errors: VAB has been reported to decrease the rate of sampling errors10. CNB carries a greater risk of inadequate sampling, which could potentially lead to an incomplete representation of the disease’s extent and consequently, a higher chance of false negatives 11.
- Management of High-Risk Lesions: VAB improves the management of high-risk lesions by reducing underestimation rates, particularly for certain B3 lesions, such as Lobular Neoplasia (LN), Flat Epithelial Atypia (FEA), radial scar, and papillary lesions, when specific criteria are met. However, it is important to note that surgical excision remains the primary treatment approach for Atypical Ductal Hyperplasia (ADH) 12,13.
Patient and Findings Selection for VAB
The decision to perform a VAB on patients and target lesions is based on various factors, including the radiographic features of the lesion, the patient’s clinical history, and the limitations associated with alternative biopsy techniques. VAB is frequently utilized for lesions demonstrating specific characteristics, such as microcalcifications, architectural distortions, and other radiographically suspicious findings that may pose challenges for sampling with CNB 1.
Some specific indications for VAB:
- The decision to perform a VAB on patients and target lesions is based on various factors, including the radiographic features of the lesion, the patient’s clinical history, and the limitations associated with alternative biopsy techniques. VAB is frequently utilized for lesions demonstrating specific characteristics, such as microcalcifications, architectural distortions, and other radiographically suspicious findings that may pose challenges for sampling with CNB 1.
- Lesions with suspicious or indeterminate imaging features, such as microcalcifications, architectural distortions, or asymmetries, where VAB can help determine the need for further treatment or surgery 15–17.
- Known or suspected high-risk lesions, such as atypical ductal hyperplasia (ADH), atypical lobular hyperplasia (ALH), lobular carcinoma in situ (LCIS), radial scar, or papillary lesions, to evaluate the extent of the lesion and exclude the presence of malignancy 12.
- Reassessing previously benign biopsies with persistent imaging-pathology discordance, interval changes, or inconclusive initial results due to insufficient sampling 18,19
- Monitoring of neoadjuvant chemotherapy response in breast cancer patients by performing VAB after treatment, showed promising early results, pending further trials to better define protocols and future guidelines 20–22.
Image Guidance Modality
VAB can be performed using different image guidance modalities, depending on the specific clinical scenario and available equipment. The commonly utilized mammographic guidance modalities for VAB include ultrasound conventional stereotaxy and digital breast tomosynthesis (DBT)-guided biopsy. Conventional stereotaxy involves obtaining two-dimensional mammography images from different angles to localize the lesion in three-dimensional space. The stereotactic unit uses these images to guide the biopsy needle to the target lesion, demonstrating high diagnostic accuracy rates and widespread use for VAB guidance 23,24.
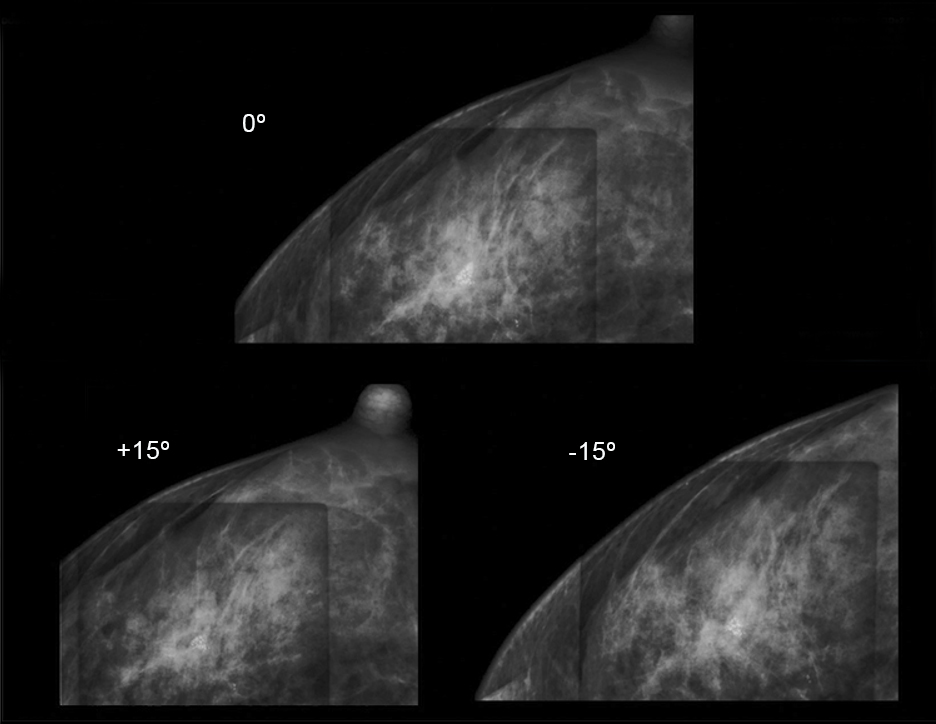
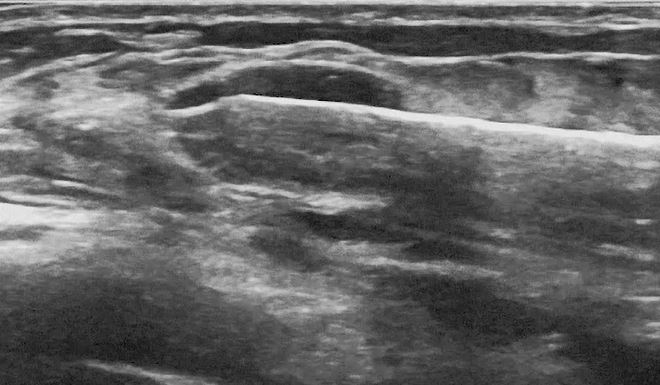
Photos used with the express permission of the author
Tomosynthesis or DBT-guided biopsy utilizes digital breast tomosynthesis, capturing multiple low-dose mammography images from various angles to create a three-dimensional image of the breast. This modality has shown promise in improving diagnostic accuracy and reducing the need for additional imaging or biopsy procedures 25–27.
Tomosynthesis guidance
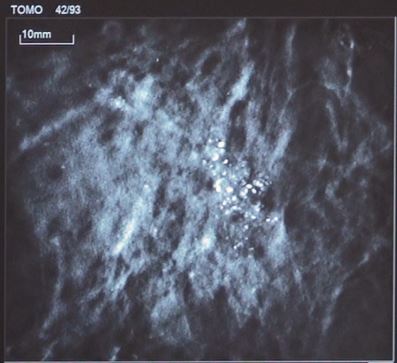
Photo used with the express permission of the author
In addition to mammographic guidance modalities, MRI guidance can be employed for VAB in cases where the MRI findings indicate the need for a biopsy due to a lack of correlation with other imaging techniques. MRI guidance allows for precise targeting and effective sampling of these specific findings 28–30.
MRI guidance
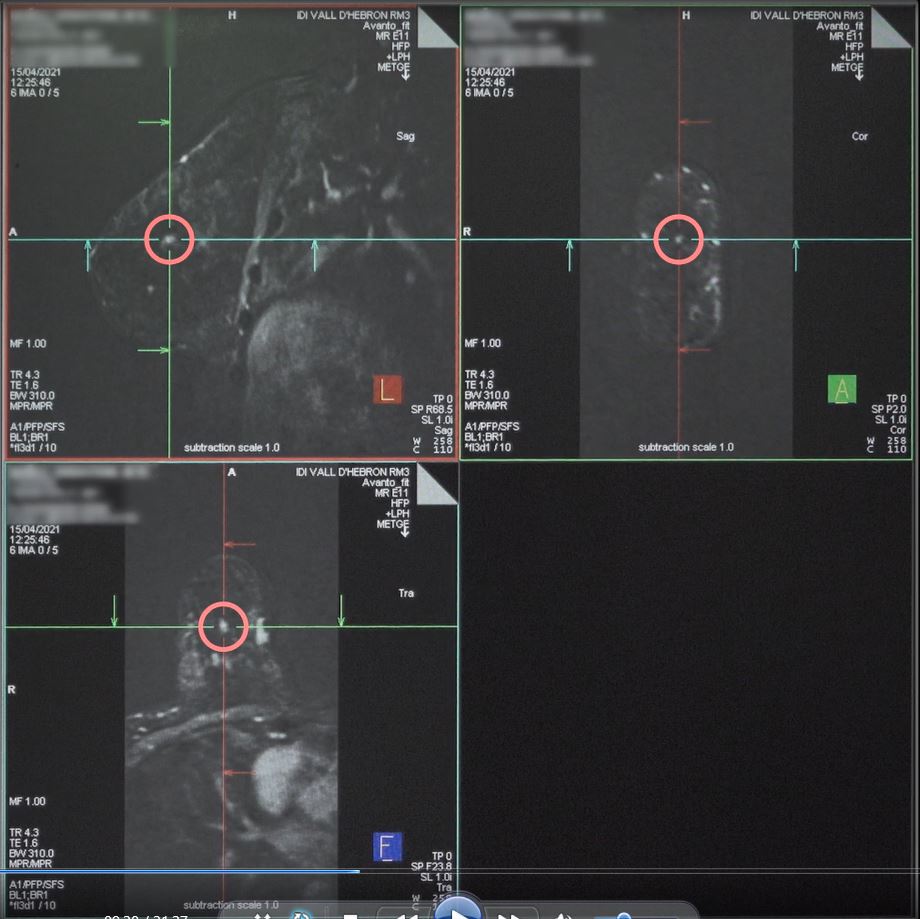
Photo used with the express permission of the author
Furthermore, a contrast-enhanced mammography-guided biopsy (CEM-guided biopsy or contrast-enhanced stereotaxy) has been implemented, initially for indications similar to MRI 31. This modality involves the administration of an iodinated contrast agent, through intravenous injection, to enhance the visualization of the lesion and surrounding tissues on mammography images. A CEM-guided biopsy can help identify small or subtle lesions and improve the accuracy of VAB by enhancing lesion localization and reducing the chance of sampling error.
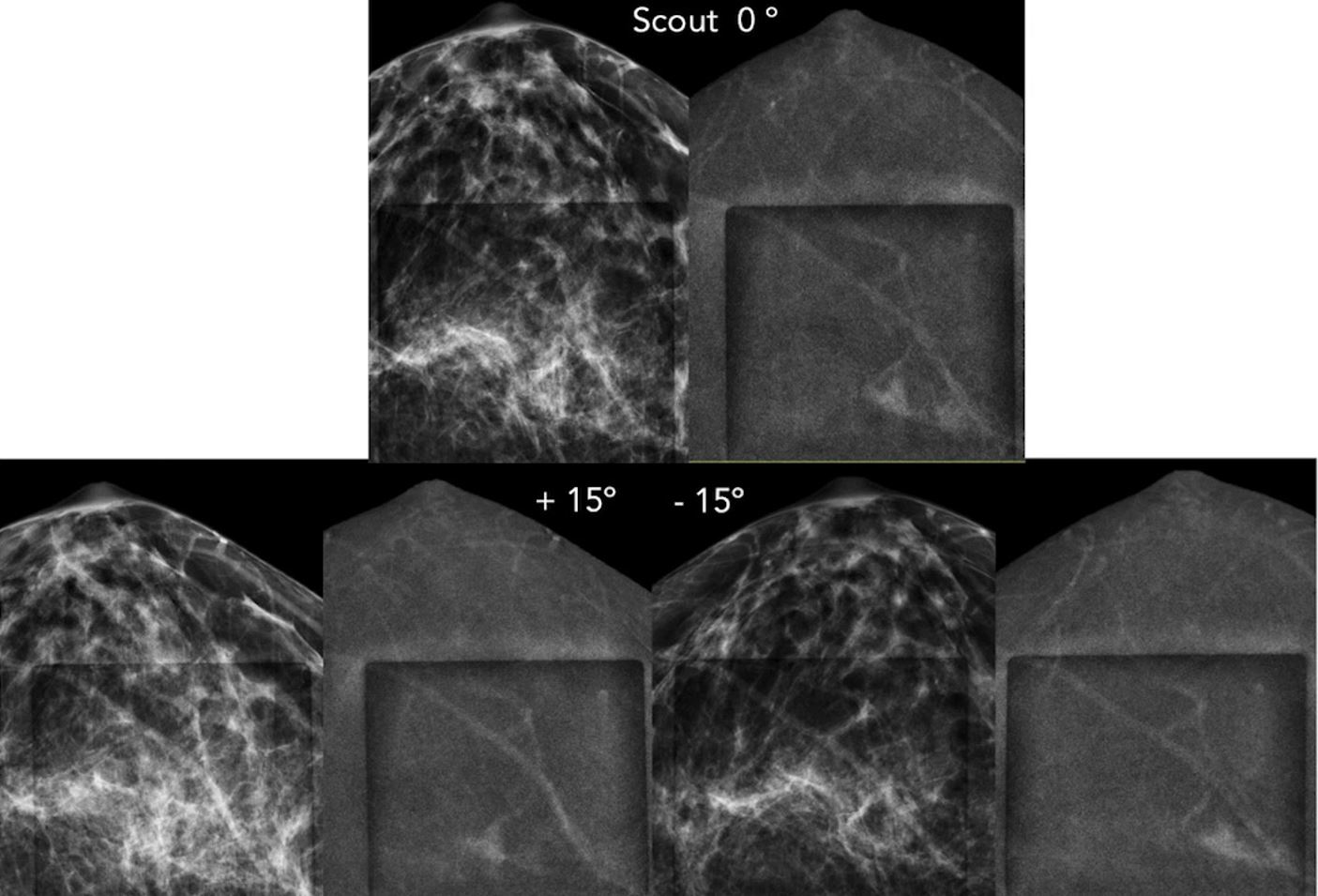
Photo used with the express permission of the author
The choice of the most appropriate mammographic guidance modality depends on the specific clinical scenario, characteristics of the lesion, and equipment availability. The goal is to achieve accurate lesion localization, optimal sampling, and minimize patient discomfort and radiation exposure.
Needle Gauges and Sample Sizes
Needle gauge and size are vital determinants in VAB procedures, available typically from 7G to 14G. The choice hinges on several factors, such as lesion attributes (size and location), patient considerations, and the operator’s preference.
Compared to CNB, VAB usually yields larger sample sizes for pathological assessment. Larger diameter needles (smaller gauge, 7G to 8G), are typically used for lesions requiring a higher volume of tissue sample, whether for diagnosis or excision purposes. Conversely, smaller diameter needles (larger gauge, 10G to 14G) provide lower amounts of tissue. Under certain circumstances, a 14G needle, the smallest VAB gauge available, might be chosen for lesions near critical structures to mitigate risk.
Surgical biopsy traditionally involves an average of 20 g sample size. Given that VAB often yields samples around 5-10 g, with a more targeted acquisition due to image guidance, some studies suggest that its sensitivity could be comparable to surgical biopsy 32. Earlier research indicates that the 11G probe collects around 100 mg of tissue per procedure, while the 14G core probe collects approximately 15 mg per sample 33. The 7G needle, currently the broadest diameter option available, is known to obtain more substantial sample sizes [2]. However, other elements such as the needle’s structure, the strength of the suction, and the suction’s duration also play a role in determining the sample size. Similarly, the hardness of the lesion targeted for biopsy also impacts the sample collection process. VAB can obtain larger samples from softer lesions than harder ones 33. Despite the lack of consensus on the sample amount, common practice suggests collecting over 10-12 specimens using a 10-11G needle or 4-6 specimens using a 7-8G needle.
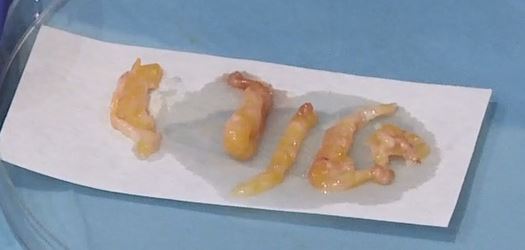
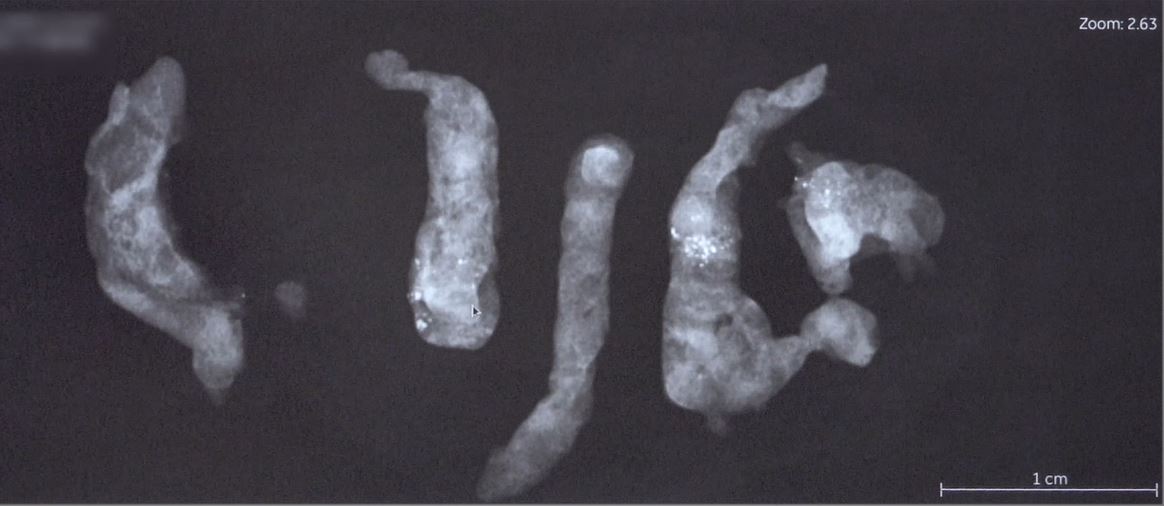
Clip Placement and Radiological Markers
Clip placement is a crucial aspect of VAB procedures, particularly in cases where the targeted lesion is otherwise occult or may faintly appear or disappear after the biopsy procedure. Radiological markers, such as tissue markers or clips, are used to localize the biopsy site and provide important reference points for future imaging and follow-up 34.
Radiological markers serve multiple purposes in VAB:
- Localization: Placing a radiological marker, typically a clip, at the biopsy site, ensures accurate localization of the area where the tissue sample was obtained. This is particularly important when the lesion is not readily visible on imaging or when there is concern about its visibility post-biopsy.
- Follow-up and Surveillance: The presence of a radiological marker allows for easier identification of the biopsy site during follow-up imaging. It provides a reference point for monitoring the area of interest and assessing changes over time. The marker aids in distinguishing between post-biopsy changes and any potential residual or recurrent lesions.
- Surgical Guidance: In cases where VAB results indicate the need for surgical excision, the placement of a radiological marker facilitates precise localization of the targeted area during subsequent surgical procedures. This ensures that the surgical team can accurately identify the site of concern and perform the necessary excision with minimal tissue removal.
Radiological markers used in VAB procedures are typically small, inert clips made of materials that are visible on imaging, such as titanium or stainless steel. These clips are carefully placed under imaging guidance, ensuring accurate positioning relative to the targeted lesion. The radiological markers are generally well-tolerated by the patient and do not interfere with subsequent imaging or treatment 34,35. The main complication is marker migration after deployment 36–38 and therefore is important to assess adequate positioning in post-procedure mammography.
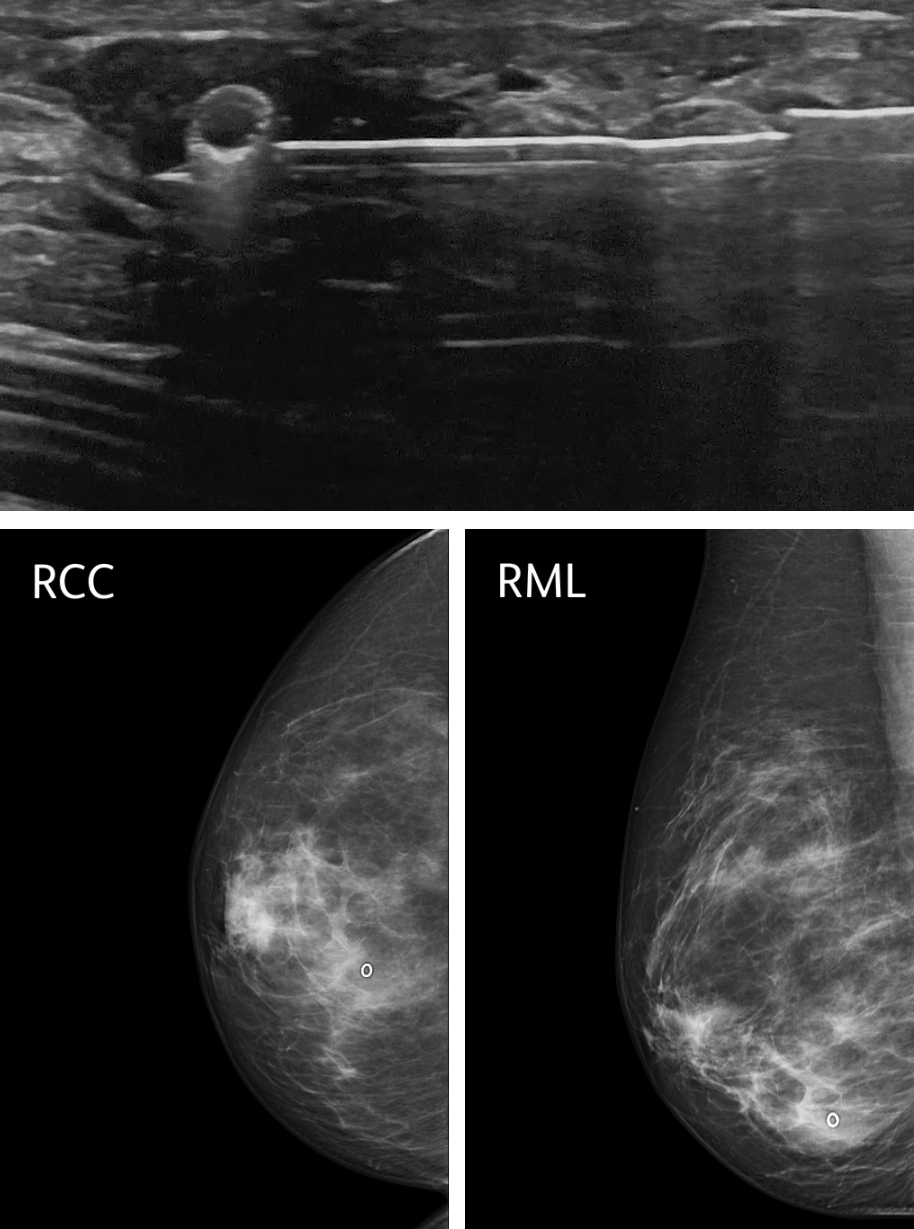
Photos used with the express permission of the author
Complications of VAB
VAB is a safe and minimally invasive procedure, with a low risk of complications. The most common complications include skin tear, pain, bruising, and hematoma at the biopsy site, which usually resolves spontaneously within a few days. The incidence of serious complications, such as bleeding, infection, or pneumothorax, is rare and seen in only 0.3% of patients 39.
To minimize the risk of complications, careful patient selection, appropriate needle size, and proper technique are essential. A thorough evaluation of the patient’s coagulation status and the use of local anesthesia with epinephrine can also help reduce the risk of bleeding 40. Additionally, while performing the biopsy, it is important to ensure accurate placement of the needle and to monitor for potential complications in real-time.
In cases of bleeding or hematoma, compression and observation are often sufficient for management. However, if the bleeding is persistent or severe, additional interventions such as embolization or surgical exploration may be necessary. Infection can be treated with antibiotics, and in rare cases, abscess drainage may be required.
Nipple discharge, an unusual complication, can often be managed conservatively, but persistent or suspicious discharge may require further evaluation.
Another potential complication of VAB is the development of biopsy-related artifacts on subsequent mammography, which can mimic distortions or calcifications and potentially lead to unnecessary additional procedures, although this is not usually a problem. To minimize the risk of equivocal interpretation, it is important to carefully document the biopsy location and technique.
To minimize the risk of complications, including skin tears or hematomas, during the VAB procedure, it is essential to exercise caution and take necessary precautions. The combination of the vacuum system and compression from the ultrasound probe can potentially lead to a skin tear. Therefore, maintaining clear visualization of the needle and utilizing ultrasound guidance throughout the procedure are crucial steps. Adequate local anaesthesia should also be provided to ensure patient comfort. By adhering to these measures, the aim is to prevent complications and ensure the safe and successful completion of the VAB procedure.
Conclusion
VAB is a widely accepted minimally invasive diagnostic tool for breast interventions. It offers advantages such as improved accuracy, larger sample sizes, and reduced sampling errors compared to traditional biopsy methods. The appropriate patient selection and imaging guidance modality are critical to ensure optimal sampling and minimize complications. VAB can be performed under different image guidance modalities, including conventional stereotaxy, digital breast tomosynthesis (DBT)-guided biopsy, MRI guidance, and contrast-enhanced mammography-guided biopsy (CEM-guided biopsy). Needle size and gauge should be chosen based on lesion characteristics and patient factors, with larger needles preferred for larger lesions or those requiring more tissue sampling. Complications of VAB are rare and usually minor, and the procedure is generally well-tolerated by patients. VAB has revolutionized breast biopsy, enabling accurate diagnoses with minimal tissue damage and improved procedure outcomes.
References
1. Fornage BD. Vacuum-Assisted Biopsy. Interventional Ultrasound of the Breast. Published online 2020:317-325. doi:10.1007/978-3-030-20829-5_13
2. O’Flynn EAM, Wilson ARM, Michell MJ. Image-guided breast biopsy: state-of-the-art. Clin Radiol. 2010;65(4):259-270. doi:10.1016/j.crad.2010.01.008
3. Westenend PJ, Sever AR, Beekman-De Volder HJC, Liem SJ. A Comparison of Aspiration Cytology and Core Needle Biopsy in the Evaluation of Breast Lesions.
4. Parker SH, Burbank F, Jackman RJ, et al. Percutaneous Large-Core Breast Biopsy: A Multi-Institutional Study. Radiology 193.2 (1994): 359-364
5. Burbank F. Stereotactic breast biopsy of atypical ductal hyperplasia and ductal carcinoma in situ lesions: improved accuracy with directional, vacuum-assisted biopsy. Radiology. Published online March 1997:843-847. doi:10.1148/radiology.202.3.9051043
6. Bennett IC, Saboo A. The Evolving Role of Vacuum Assisted Biopsy of the Breast: A Progression from Fine-Needle Aspiration Biopsy. World J Surg. 2019;43(4):1054-1061. doi:10.1007/s00268-018-04892-x
7. Fred Burbank M.D. Stereotactic Breast Biopsy History Present and Its Future. Am Surg. 1996;62.
8. Suh YJ, Kim MJ, Kim EK, et al. Comparison of the underestimation rate in cases with ductal carcinoma in situ at ultrasound-guided core biopsy: 14-gauge automated core-needle biopsy vs 8- or 11-gauge vacuum-assisted biopsy. Br J Radiol. 2012;85(1016):e349. doi:10.1259/BJR/30974918
9. Bohan S, Ramli Hamid MT, Chan WY, et al. Diagnostic accuracy of tomosynthesis-guided vacuum assisted breast biopsy of ultrasound occult lesions. Scientific Reports 2021 11:1. 2021;11(1):1-13. doi:10.1038/s41598-020-80124-4
10. Wang ZL, Liu G, Li JL, et al. Breast lesions with imaging-histologic discordance during 16-gauge core needle biopsy system: Would vacuum-assisted removal get significantly more definitive histologic diagnosis than vacuum-assisted biopsy? Breast Journal. 2011;17(5):456-461. doi:10.1111/j.1524-4741.2011.01128.x
11. Lacambra MD, Lam CC, Mendoza P, et al. Biopsy sampling of breast lesions: Comparison of core needle- and vacuum-assisted breast biopsies. Breast Cancer Res Treat. 2012;132(3):917-923. doi:10.1007/s10549-011-1639-3
12. Cullinane C, Byrne J, Kelly L, O Sullivan M, Antony Corrigan M, Paul Redmond H. The positive predictive value of vacuum assisted biopsy (VAB) in predicting final histological diagnosis for breast lesions of uncertain malignancy (B3 lesions): A systematic review & meta-analysis. European Journal of Surgical Oncology. 2022;48(7):1464-1474. doi:10.1016/J.EJSO.2022.04.005
13. Rageth CJ, Rubenov R, Bronz C, et al. Atypical ductal hyperplasia and the risk of underestimation: tissue sampling method, multifocality, and associated calcification significantly influence the diagnostic upgrade rate based on subsequent surgical specimens. Breast Cancer. 2019;26(4):452-458. doi:10.1007/s12282-018-00943-2
14. Park HL, Kim LS. The current role of vacuum assisted breast biopsy system in breast disease. J Breast Cancer. 2011;14(1):1-7. doi:10.4048/jbc.2011.14.1.1
15. Ambinder EB, Plotkin A, Euhus D, et al. Tomosynthesis-guided vacuum-assisted breast biopsy of architectural distortion without a sonographic correlate: A retrospective review. American Journal of Roentgenology. 2021;217(4):845-854. doi:10.2214/ajr.20.24740
16. Charmi Vijapura, Yang L, Xiong J, Fajardo LL. Imaging Features of Nonmalignant and Malignant Architectural Distortion Detected by Tomosynthesis. American Journal of Roentgenology. 2018;211(6):1397-1404. doi:https://doi.org/10.2214/ajr.18.19658
17. van Bekkum S, Dams FEM, Westenend PJ, van Rosmalen J, Menke-Pluijmers MBE, Kock MCJM. The reassurance of the diagnosis benign calcifications after vacuum-assisted stereotactic breast biopsy. Breast Journal. 2021;27(8):681-683. doi:10.1111/tbj.14237
18. Li JL, Wang ZL, Su L, Liu XJ, Tang J. Breast lesions with ultrasound imaging-histologic discordance at 16-gauge core needle biopsy: Can re-biopsy with 10-gauge vacuum-assisted system get definitive diagnosis? Breast. 2010;19(6):446-449. doi:10.1016/j.breast.2010.04.003
19. Jörg I, Wieler J, Elfgen C, et al. Discrepancies between radiological and histological findings in preoperative core needle (CNB) and vacuum-assisted (VAB) breast biopsies. J Cancer Res Clin Oncol. 2021;147(3):749-754. doi:10.1007/S00432-020-03481-7/FIGURES/4
20. Koelbel V, Pfob A, Schaefgen B, et al. Vacuum-Assisted Breast Biopsy After Neoadjuvant Systemic Treatment for Reliable Exclusion of Residual Cancer in Breast Cancer Patients. Ann Surg Oncol. 2022;29(2):1076-1084. doi:10.1245/S10434-021-10847-9/FIGURES/2
21. Tasoulis MK, Lee HB, Yang W, et al. Accuracy of Post–Neoadjuvant Chemotherapy Image-Guided Breast Biopsy to Predict Residual Cancer. JAMA Surg. 2020;155(12):e204103-e204103. doi:10.1001/JAMASURG.2020.4103
22. Kuerer HM, Smith BD, Krishnamurthy S, et al. Eliminating breast surgery for invasive breast cancer in exceptional responders to neoadjuvant systemic therapy: a multicentre, single-arm, phase 2 trial. Lancet Oncol. 2022;23(12):1517-1524. doi:10.1016/S1470-2045(22)00613-1
23. Jackman RJ, Burbank F, Parker SH, et al. Stereotactic Breast Biopsy of Nonpalpable Lesions: Determinants of Ductal Carcinoma in Situ Underestimation Rates. Radiology. 2001;218(2):497-502. doi:10.1148/radiology.218.2.r01fe35497
24. Huang ML, Adrada BE, Candelaria R, Thames D, Dawson D, Yang WT. Stereotactic breast biopsy: Pitfalls and pearls. Tech Vasc Interv Radiol. 2014;17(1):32-39. doi:10.1053/j.tvir.2013.12.006
25. Omofoye TS, Martaindale S, Teichgraeber DC, Parikh JR. Implementation of Upright Digital Breast Tomosynthesis-guided Stereotactic Biopsy. Acad Radiol. 2017;24(11):1451-1455. doi:10.1016/j.acra.2017.05.010
26. Ariaratnam NS, Little ST, Whitley MA, Ferguson K. Digital breast Tomosynthesis vacuum assisted biopsy for Tomosynthesis-detected Sonographically occult lesions. Clin Imaging. 2018;47:4-8. doi:10.1016/j.clinimag.2017.08.002
27. Schrading S, Distelmaier M, Dirrichs T, et al. Digital Breast Tomosynthesis–guided Vacuum-assisted Breast Biopsy: Initial Experiences and Comparison with Prone Stereotactic Vacuum-assisted Biopsy. Radiology. Published online 2015. doi:10.1148/radiol.14141397
28. McGrath AL, Price ER, Eby PR, Rahbar H. MRI-guided breast interventions. Journal of Magnetic Resonance Imaging. 2017;46(3):631-645. doi:10.1002/jmri.25738
29. Rauch GM, Dogan BE, Smith TB, Liu P, Yang WT. Outcome analysis of 9-gauge MRI-guided vacuum-assisted core needle breast biopsies. American Journal of Roentgenology. 2012;198(2):292-299. doi:10.2214/AJR.11.7594
30. Imschweiler T, Haueisen H, Kampmann G, et al. MRI-guided vacuum-assisted breast biopsy: Comparison with stereotactically guided and ultrasound-guided techniques. Eur Radiol. Published online 2014. doi:10.1007/s00330-013-2989-5
31. Alcantara R, Posso M, Pitarch M, et al. Contrast-enhanced mammography-guided biopsy: technical feasibility and first outcomes. Eur Radiol. Published online July 27, 2022. doi:10.1007/s00330-022-09021-w
32. Pieri A, Hemming D, Westgarth J, Lunt L. Vacuum-assisted biopsy is a viable alternative to surgical biopsy in the investigation of breast lesions of uncertain malignant potential. Surgeon. 2017;15(2):59-64. doi:10.1016/j.surge.2015.10.001
33. Nakano S, Imawari Y, Mibu A, Otsuka MH, Oinuma T. Differentiating vacuum-assisted breast biopsy from core needle biopsy: Is it necessary? Br J Radiol. 2018;91(1092). doi:10.1259/BJR.20180250
34. Thomassin-Naggara I, Lalonde L, David J, Darai E, Uzan S, Trop I. A plea for the Biopsy marker: How, why and why not clipping after breast biopsy? Breast Cancer Res Treat. 2012;132(3):881-893. doi:10.1007/s10549-011-1847-x
35. Shalaby LASED, Khallaf ES el din, Moussa MM. Clip and wire localization of locally advanced malignant breast masses in patients undergoing neoadjuvant chemotherapy and breast conservation therapy. Egyptian Journal of Radiology and Nuclear Medicine. 2019;50(1):1-9. doi:10.1186/S43055-019-0066-Z/TABLES/3
36. Jain A, Khalid M, Qureshi MM, et al. Stereotactic core needle breast biopsy marker migration: An analysis of factors contributing to immediate marker migration. Eur Radiol. 2017;27(11):4797-4803. doi:10.1007/s00330-017-4851-7
37. Le-Petross HT, Hess KR, Knudtson JD, et al. Effect of Mammography on Marker Clip Migration After Stereotactic-Guided Core Needle Breast Biopsy. Curr Probl Diagn Radiol. 2017;46(6):410-414. doi:10.1067/j.cpradiol.2017.02.001
38. Esserman LE, Cura MA, DaCosta D. Recognizing Pitfalls in Early and Late Migration of Clip Markers after Imaging-guided Directional Vacuum-assisted Biopsy. Radiographics. 2004;24(1):147-156. doi:10.1148/rg.241035052
39. Leng Leng Young Lin, Yiming Gao, Alana A. Lewin, Hildegard K. Toth, Samantha L. Heller and LM. Overstated Harms of Breast Cancer Screening? A Large Outcomes Analysis of Complications Associated With 9-Gauge Stereotactic Vacuum-Assisted Breast Biopsy. AJR Am J Roentgenol. 2019;212:4(April):925-932. 10.2214/AJR.18.20421
40. Li X, Trerotola SO. Local Anesthesia in Interventional Radiology. Semin Intervent Radiol. 2022;39(4):381-386. doi:10.1055/s-0042-1757342
Guidelines+
Wallis M, Tarvidon A, Helbich T, Schreer I. Guidelines from the European Society of Breast Imaging for diagnostic interventional breast procedures. European Radiology. 2006;17(2):581-588. doi:https://doi.org/10.1007/s00330-006-0408-x
Bick U, Trimboli RM, Athanasiou A, et al. Image-guided breast biopsy and localisation: recommendations for information to women and referring physicians by the European Society of Breast Imaging. Insights into Imaging. 2020;11(1). doi:https://doi.org/10.1186/s13244-019-0803-x
Clinical procedures+
Tomosynthesis Biopsy exam
Biopsy Ultrasound Clinical Procedures
MRI guided VAB Clinical Procedure

